
纳米颗粒药物研发态势报告
利用汤森路透Cortellis数据库检索纳米颗粒药物信息,从数据的角度揭示当前纳米颗粒药物领域的进展和趋势。其中药物信息包括Biosimilar,涉及成熟API的创新剂型产品或给药系统;不包含仿制药,OTC,治疗装置,非治疗用制品,兽药。共计检索到纳米药物信息1715个,其中活跃药物959个。以下针对959个活跃药物进行分析。
一、国际纳米颗粒药物研发趋势
1.研发国家/地区
药物研发比较活跃的TOP5国家/地区分别是:US(649)、UK(74)、Canada(63)、China(59)、Japan(48)。
其中中国地区共59个药物,国内企业研发的药物为45个,其他国家或地区在中国的药物研发情况分别是US(10)、UK(4)、Greece(1)、HongKong(1)、Japan(1)、Switzerland(1)。
2.研发机构
共有705个机构从事纳米颗粒药物研发,包括新兴技术企业、大型企业、科研院所等不同类型。
美国的Aphios公司共有18个在研药物,是该领域主要的研发者。国内共有41个机构从事纳米颗粒药物研发,北京康乐卫士生物技术股份有限公司共有4个在研药物,是药物数量最多的机构(见图2、3)。
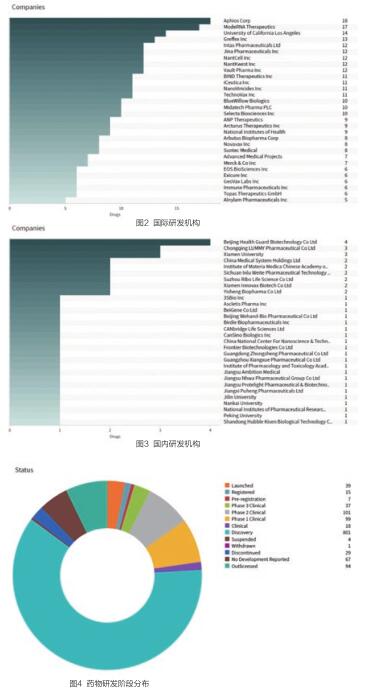
3.研发阶段分布
纳米颗粒研发药物中,上市药物为39个,注册阶段的药物为15个,预注册阶段的药物有7个;临床阶段的药物有203个;临床前研发状态阶段的药物为801项;其他162个为终止或暂停研究的项目(见图4)。
4.适用病症
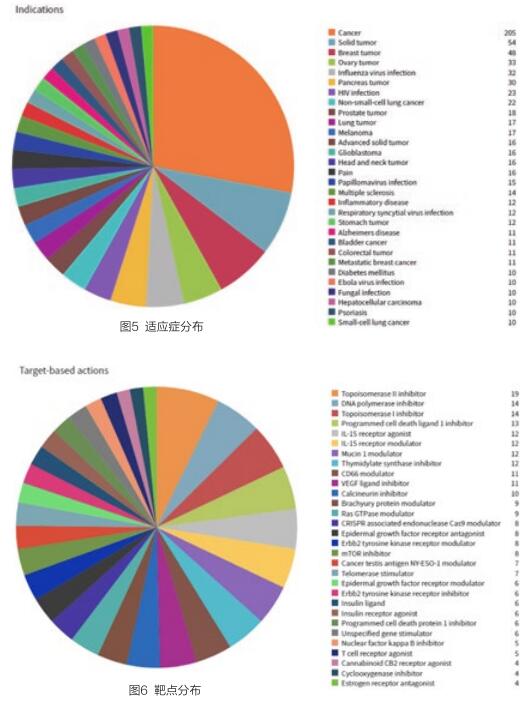
纳米颗粒药物的适用病症以癌症为主,实体肿瘤、乳腺肿瘤、卵巢肿瘤是主要的治疗领域、流感病毒感染等是主要的适用病症(见图5)。
5.靶点分布
活跃药物的纳米颗粒药物的药物靶点共涉及406个,以拓扑异构酶II抑制剂、DNA聚合酶抑制剂、拓扑异构酶I抑制剂、程序性细胞死亡配体1抑制剂等靶点的药物研发项目为多(见图6)。
二、药物销售分析
其中18个药物具有药物销售及预测数据,这17个药物的信息如下:
Patisiran
Highest Status: Launched
Last Change Date: 09-Jul-2019
Alnylam has developed and launched patisiran (Onpattro; ALN-TTR02; GZ-438027; SAR-438027), a second-generation siRNA therapy using the MC3 lipid that targets the transthyretin (TTR) gene (both wild-type and all known mutant forms of TTR, including the V30M and V122I) delivered using Arbutus' (previously Tekmira) lipid nanoparticle technology [2062488], [2062828]. In the US, the drug is indicated for the polyneuropathy of hereditary TTR-mediated amyloidosis (hATTR) in adults [2062523]. In the
irinotecan sucrosofate (nanoliposomal, cancer),
PharmaEngine/Merrimack/Shire
Highest Status: Launched
Last Change Date: 09-Jul-2019
Ipsen, following its acquisition of the drug from Merrimack Pharmaceuticals (formerly HERMES Biosciences), and licensees Servier, following its acquisition of the drug from Shire, following its merger with Baxalta (previously a spin-out of Baxter International), and Pharma Engine have developed and launched MM-398 (Onivyde; PEP-02; nal-IRI; BAX-2398; SHP-673), an encapsulated nanoliposomal formulation of irinotecan sucrosofate [1184015], [1189837], [1218064], [1596725], [1665788], [1674744]
vincristine sulfate (liposomal injection), Spectrum Pharmaceuticals
Highest Status: Launched
Last Change Date: 09-Jul-2019
Acrotech BioPharma , under license from Spectrum Pharmaceuticals (which itself gained rights following the acquisition of Talon Therapeutics; previously called Hana Biosciences), under license from Tekmira (now Arbutus, formerly Inex), has developed and launched Marqibo (Onco-TCS), an injectable liposomal formulation (2.25 mg/m 2 ) of the tubulin polymerization inhibitor vincristine sulfate, which utilizes Inex's transmembrane carrier system (TCS) Optisome [1473034], [344464], [1453482]
Docetaxel
Highest Status: Launched
Last Change Date: 08-Jul-2019
Rhone-Poulenc Rorer (subsequently Aventis, then sanofi-aventis, now Sanofi ) has developed and launched the antineoplastic taxoid docetaxel (Taxotere). In the US, the drug is indicated for the treatment of locally advanced or metastatic breast cancer after failure of prior chemotherapy, and in combination with doxorubicin and cyclophosphamide for the adjuvant treatment of operablenode-positive breast cancer; as a monotherapy for the treatment of locally advanced or metastatic non-small-cell
paclitaxel (albumin-bound nanoparticle,intravenous), Celgene
Highest Status: Launched
Last Change Date: 08-Jul-2019
A b r a x i s B i o S c i e n c e ( f o r m e r l y American BioScience (ABI), then American Pharmaceutical Partners (APP); now Celgene), using its proprietary nab technology, and various licensees have developed and launched ABI-007 (Abraxane; Abraxus; Capxol; nab-paclitaxel), a nanoparticle, albumin-bound suspension formulation of paclitaxel that is protein stable and Cremophor free [1172389], [1612582]. The product is indicated in the US for the treatment of breast cancer after failure of combination chemotherapy
RSV-F
Highest Status: Phase 3 Clinical
Last Change Date: 04-Jul-2019
Novavax is developing a virus-like particle (VLP)-based recombinant nanoparticle vaccine, RSV-F (ResVax), targeting the viral fusion protein, using technology licensed from the University of Massachusetts Medical School (UMMS), for the potentialim prevention of respiratory syncytial virus (RSV) infection [961432], [1273947], [1329491], [2061523]. In November 2015, a phase III trial (RESOLVE) was initiated in the US in elderly subjects [1711564]; in September 2016, negative topline data
Inclisiran
Highest Status: Phase 3 Clinical
Last Change Date: 21-Jun-2019
The Medicines Company (TMC), under license from Alnylam Pharmaceuticals, is developing inclisiran (ALN-PCSsc; ALN-60212; PCSK9si), the lead from a series of siRNAs that silence the gene for proprotein convertase subtilisin/ kexin type 9 (PCSK9), formulated using Tekmira's MC3 lipid nanoparticles and delivered using the Enhanced Stabilization Chemistry(ESC)-GalNAc-siRNA conjugate platform, for the potential sc treatment of hypercholesterolemia [678687], [1365733], [1337825], [1490429], [1484088]
Doravirine
Highest Status: Registered
Last Change Date: 20-Jun-2019
Merck & Co has developed doravirine (MK-1439; Pifeltro), a next-generation non-nucleoside reverse transcriptaseinhibitor, as a once-daily oral film-coatedtablet formulation [1238852], [1310568], [2068014]. Doravirine is indicated in the US in combination with other antiretroviralagents for the treatment of HIV-1 infectionin adult patients with no prior antiretroviral treatment history [2068111]. In the EU the product is indicated for the treatment of HIV-1 infection in adults with
dantrolene sodium (heat stroke/malignant hyperthermia), Eagle Pharmaceuticals
Highest Status: Launched
Last Change Date: 17-Jun-2019
Eagle Pharmaceuticals has developed and launched Ryanodex (EP-4104; EGL-4104; EGL-4104-C-1702), a nanoparticle suspension formulation of dantrolene sodium, which acts to inhibit the excitation-contraction coupling process in skeletal muscle, utilizing sublicensed nanocrystal technology from Lyotropic [1539064]. The product is indicated in the US for the treatment of malignant hyperthermia in conjunction with appropriate supportive measures and for the prevention of malignant hyperthermia in
aripiprazole (extended-release intramuscular, LinkeRx, schizophrenia), Alkermes
Highest Status: Launched
Last Change Date: 30-May-2019
Alkermes has developed and launched aripiprazole lauroxil (ALKS-9070; ALKS-9072; Aristada; Aristada Initio), an extended-release formulation of the partial dopamine D2 and 5-HT 1a receptor agonist and 5-HT 2a receptor antagonist aripiprazole, using its LinkeRx technology platform [1073130], [1682099], [1707743]. The product is indicated in the US for the treatment of schizophrenia and is available in four dosing options 441, 662, 882 and 1064 mg for once monthly, 6-weekly and 2-monthly
paliperidone palmitate
Highest Status: Launched
Last Change Date: 27-May-2019
Johnson & Johnson (J&J), through its subsidiaries Ortho-McNeil-Janssen (now Janssen Pharmaceuticals ) and Janssen-Cilag, has developed and launched Invega Sustenna(Xeplion, Sustenna), a long-acting 4-weekim depot formulation of the dual dopamine D2 and 5HT antagonist paliperidone palmitate, an ester of the active ingredient in paliperidone ER (Invega) and a metabolite of risperidone [500260], [1197110], [1396148]. The product utilizes Elan's NanoCrystal technology, and is indicated
cabotegravir (long-acting injectable), ViiV
Highest Status: Phase 3 Clinical
Last Change Date: 22-May-2019
ViiV Healthcare (formed from the merger of Pfizer and GlaxoSmithKline's (GSK) HIV assets) is developing cabotegravir LA (S-265744 LAP, S-1265744 LAP, GSK-1265744 LAP; GSK-744 LAP; S/GSK-1265744 LAP), a long-acting, nanocrystal, injectable formulation of ViiV's oral HIV integrase inhibitor cabotegravir, for the potential treatment and pre-exposure prophylaxis of HIV infection [1054205]; [1146833], [1630387], [1643398]. In February 2016, the company was seeking to outlicense the program [1731014]
vancomycin hydrochloride (dry-powder inhaled, MRSA infection), Savara
Highest Status: Phase 3 Clinical
Last Change Date: 14-May-2019
Savara is developing SAV-005 (Aero Vanc), a once-or twice-daily dry powder inhaled formulation of the glycopeptide antibiotic vancomycin hydrochloride, developed using its nanocluster dry-powder formulation technology, for the potential treatment of pulmonary MRSA infection in patients with cystic fibrosis (CF) [1229078], [1229091], [1340304]. In September 2017, a phase III trial (AVAIL) was initiated in persistent MRSA infection in patients with CF [2012644], [2013035]; in February 2019
meloxicam (pain, NanoCrystal), Recro
Highest Status: Pre-registration
Last Change Date: 12-Apr-2019
Recro , though an asset acquisition from Alkermes (following a merger with Elan Drug Technologies a subsidiary of Elan), is developing N-1539, an iv or im long-acting formulation of meloxicam, a COX-2 inhibitor, created using Elan's NanoCrystal technology, for the potential relief of postoperative pain [1039330], [1230500], [1342792], [1674758]. In March 2018, the company was seeking to outlicense outside the US and potentially in the US [2011630]. In July 2017, an NDA was submitted to
Cervarix
Highest Status: Launched
Last Change Date: 06-Mar-2019
Glaxo SmithKline (GSK; formerly SmithKline Beecham, SB), under license from MedImmune (a subsidiary of AstraZeneca following a June 2007 acquisition [802396]),has developed and launched Cervarix (MEDI-517), a combination vaccine comprising self-assembling, virus-like particles (VLPs)based on HPV-16 (MEDI-503) and HPV-18 (MEDI-504) strains, formulated with the company's AS04 adjuvant [631792]. In the EU, the vaccine is indicated for the prevention of premalignant genital (cervical, vulvar)
SPK-8011
Highest Status: Phase 3 Clinical
Last Change Date: 21-Feb-2019
Spark Therapeutics (a spin-off of The Childrens Hospital of Philadelphia ), presumed to be under license from The Childrens Hospital of Philadelphia, is developing SPK-8011, a lead from its SPK-FVIII program, an adeno-associated virus (AAV) based factor VIII gene therapy comprising an AAV-LK03 (Spark200) capsid and a B-domain deleted (BDD) hFVIII transgene, developed using Selecta's nanoparticle platform, for the potential iv treatment of hemophilia A [1628935], [1490665], [1490414]
Ferumoxytol
Highest Status: Launched
Last Change Date: 19-Feb-2019
Ferumoxytol (Feraheme; Rienso; code 7228; AMI-7228) is an ultrasmall superparamagnetic iron oxide nanoparticle product that was developed by AMAG Pharmaceuticals (formerly Advanced Magnetics) and former licensee Takeda, and launched by AMAG. The product is indicated in the US for the treatment of iron deficiency anemia (IDA) in adult patients who have intolerance to oral iron or have had unsatisfactory response to oral iron or who have chronic kidney disease (CDK) [1022932]. In July 2009
megestrol acetate (oral suspension), Par
Pharmaceutical
Highest Status: Launched
Last Change Date: 20-Mar-2016
Par Pharmaceutical (via its subsidiary Strativa Pharmaceuticals) has developed and launched Megace ES, an oral suspension formulation of the progesterone derivative megestrol acetate, which utilizes Elan's NanoCrystal technology [1203393], [1203404]. The drug is indicated in the US for the treatment of anorexia, cachexia, or an unexplained significant weight loss in patients with a diagnosis of AIDS [1263181]. In July 2005, Megace ES was launched in the US [1203414]. Trials had previously
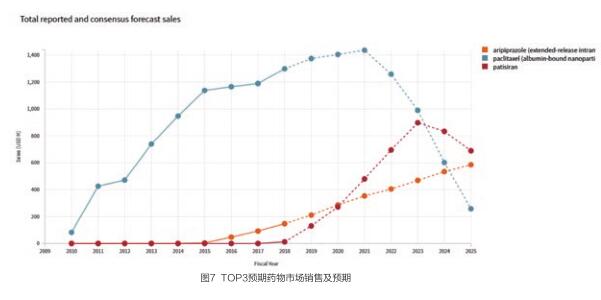
针对其中市场销售预期值最大的三个药物patisiran;paclitaxel (albumin-bound nanoparticle,intravenous), Celgene ;aripiprazole (extended-release intramuscular, LinkeRx, schizophrenia),Alkermes 查看其市场数据如下(见图7)。
三、纳米药物交易分析
1.交易机构
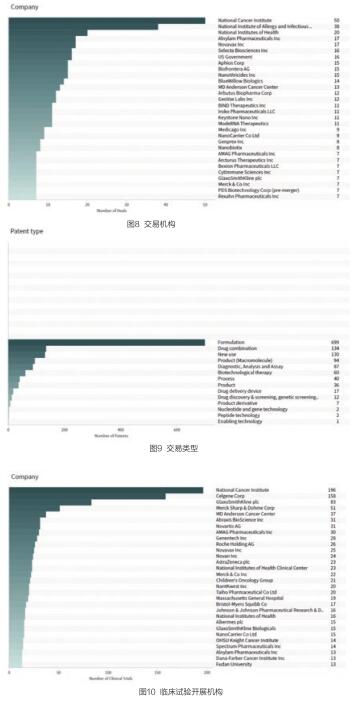
美国国家癌症研究所、美国国家过敏与传染病研究所、美国国立卫生研究院、Alnylam制药公司等是最为活跃的交易机构(见图8)。
2.交易类型
交易类型以药物的资助、早期研究/开发、开发/商业化许可证、药物开发服务等为主,其中资助是最主要的交易类型(见图9)。
四、临床试验
959个活跃药物共涉及临床试验2454项。
1. 开展机构
纳米颗粒药物临床试验的开展机构包括国家癌症研究所、Celgene公司、葛兰素史克、默克夏普和默沙东公司等(见图10)。
2. 临床试验阶段分布
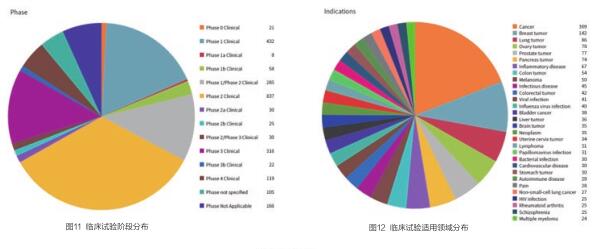
相关的2454项临床试验阶段分布如下,其中271项临床试验因为各种原因停止开展(见图11)。
3. 临床试验适用领域
癌症是临床试验最主要的适用领域,其次是乳腺肿瘤、肺肿瘤、卵巢肿瘤、前列腺肿瘤等(见图12)。
五、最新专利
共有997项专利与纳米颗粒活跃药物相关。
1. 专利权人
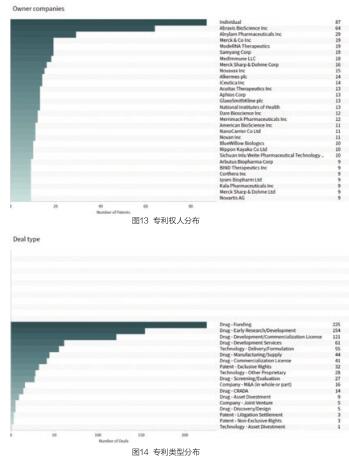
这些专利的专利权人分布如下,其中个人专利权人共有87项专利。Abraxis生物科学公司共有64项相关专利;Alnylam制药公司共有29项相关专利;默克公司和现代化RNA疗法公司分别有19项相关专利(见图13)。
2. 专利类型
这些专利涉及药物配方的专利共有699项,是专利申请最多的类型;涉及药物组合的专利共有134项;涉及药物新用途的共有130项,其他主要专利类型包括产品(大分子)、诊断和分析、生物技术治疗、工艺等方面(见图14)。
(作者单位:中科院文献情报中心咨询服务部)
 |
版权:《高科技与产业化》编辑部版权所有 京ICP备12041800号 地址:北京市海淀区中关村北四环西路33号 邮编:100080 联系电话:(010)82626611-6618 传真:(010)82627674 联系邮箱:hitech@mail.las.ac.cn |



